Describe the Energy Needed to Get a Reaction Started
What term is used to describe the minimum amount of energy required for a reaction to proceed quizlet. What is the term used to describe the energy needed to get a reaction started.

Total 2 Average 5 5 What Is The Heat Of Combustion What Is The Definition Of Enthalpy Of Combustio Heat Energy Chemical Reactions Exothermic Reaction
What is the term used to describe the energy needed to get a reactions started.

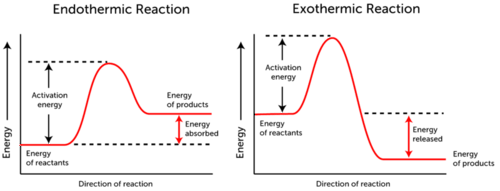
. The free energy of the reactants and products do not change just the threshold energy level needed for the reaction to commence. This means that the energy required to break the bonds in the reactants is more than the energy released when new bonds form in the products. The activation energy is the energy required to start a reaction.
A cohesion energy b chemical energy c activation energy d adhesion energy. In other words the reaction requires energy to proceed. The rate of reaction increases if the activation energy decreases.
This 27 words question was answered by Heather L. Endergonic reactions and exergonic reactions are sometimes. All chemical reactions including exothermic reactions need activation energy to get startedActivation energy is needed so reactants can move together overcome forces of repulsion and start breaking bonds.
Enzymes can lower the activation energy of a chemical reaction in three ways. The minimum amount of energy needed for a reaction to take place is called the activation energy. Enzymes are proteins that bind to a molecule or substrate to modify it and lower the energy required to make it react.
For example it takes energy to start a fire but once combustion starts the reaction releases more light and heat than it took to get it started. When a reaction starts activation energy is needed. Since its upload it has received 162 views.
The energy needed to get a reaction started is called the activation energy. Factors that affect the reaction rate include catalysts and the temperature concentration and surface area of reactants. Activation energy is the amount of energy required to initiate a reaction.
I think the correct answer from the list of choices above is option B. ACTIVATION ENERGY is the term used to describe the energy needed to get a reaction started. Hydrogen and oxygen can react to form water.
What term is used to describe the energy required to start a reaction. One of the ways the activation energy is lowered is having the enzyme bind two of the substrate molecules and orient them in a precise. How fast a reaction occurs is called the reaction rate.
O2z1qpv and 42 more users found this answer helpful. Activation energy is needed to bring reactants together so they can react. Activation energy is the term used to describe the energy needed to get a reaction started.
What is the term used to describe the energy needed to get a reaction started a. Usually once a few molecules react the rest will quickly follow. The decomposition of water into hydrogen and oxygen When water is heated to over 2000 degrees Celsius a small fraction will decompose into.
All chemical reactions even exothermic reactions need activation energy to get started. This certain energy should be reached for the reaction to proceed. The energy needed to get a reaction started is called activation energy.
What is the term used to describe the energy needed to get a reaction started. Activation energy is the energy needed to start a reaction. Some exergonic reactions also have activation energy but more energy is released by the reaction than what is required to initiate it.
The question contains content related to Chemistry and Science. On StudySoup on 5312017. The energy needed to get a reaction started is called activation energy.
The rate of reaction is given by the Arrhenius equation. What is the term used to describe the energy needed to get a reactions started. Activation Energy is the minimum amount of energy needed to start a chemical reaction.
All chemical reactions need a certain amount of activation energy to get started. Activation energy If a reaction in one direction release energy the reaction in the opposite direction. The first few reactions provide the activation energy for more molecules to react.
What is the term used to describe the energy needed to get a reaction started.

Rates Of Reaction Flashcards Flashcards Math Centers Lesson Plans

Enzymes Speed Up A Chemical Reaction By Loweing The Activation Energy Needed To Start The Reaction Description Fr Enzymes Energy Activities Chemical Reactions

Bond Energy Easy Science Chemistry Experiments Bond Flashcards

The Chemistry Of Building Better Habits James Clear Energy Activities Good Habits Chemistry

Pin By Melissa Simmons On Cookies In 2022 Biology Worksheet Enzymes Biology Scientific Method Worksheet

Studygram Calligraphy On Instagram 15 3 20 Hey Guys Sorry For My Inconsistency I Ve Finished Bio Last Semester So Thes Study Inspiration Semester Notes

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy
Activation Energy Ck 12 Foundation

Activation Energy Article Khan Academy

What Is Activation Energy Definition And Examples Energy Activities Activities Energy

Pin By Tiffanylynn20 On Schooldecor Study Notes Study Motivation Inspiration School Study Tips

Systems And Surroundings Lab In 2022 Chemistry Activities Reactions Lab

Energy Diagram Overview Parts Expii

Activation Energy Article Khan Academy

Theoretical Chemistry With Prof Mark Tuckerman Chemistry Fuel Cells Renewable Sources Of Energy

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy

Energy Diagram Overview Parts Expii

Notes On Decomposition Reaction And Its Types What Is Meant Learning Reactions

Comments
Post a Comment